- Reagents
-
- Oncology
- COVID-19
-
Pathogens
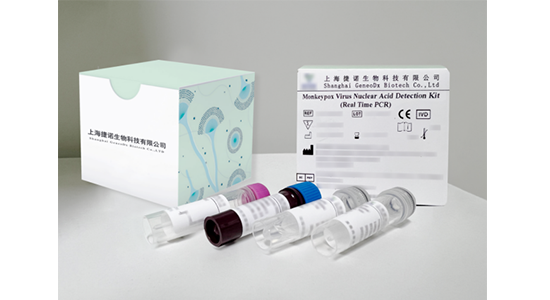
Monkeypox Virus Nucleic Acid Detection Kit (Real Time PCR) Multiplex Nucleic Acid Detection Kits for 10 STD Pathogens Multiplex Nucleic Acid Detection Kit for 18 Gastroenteritis Pathogens Multiplex Nucleic Acid Detection Kit for 22 Meningitis Pathogens Multiplex Nucleic Acid Detection Kit for 24 Respiratory Pathogens
- Fungus
- Anti-pollution
-
Introduction
This kit is used for in vitro qualitative detection of monkeypox virus F3L gene in serum samples, exudates of lesions or respiratory tract swab samples (oropharyngeal swab and nasopharyngeal swab) of suspected monkeypox cases or other cases that require monkeypox virus infection or differential diagnosis.
-
Usage
This kit is used for in vitro qualitative detection of monkeypox virus F3L gene in serum samples, exudates from lesions or respiratory tract swab samples (oropharyngeal swab and nasopharyngeal swab) of suspected monkeypox cases or other cases that require monkeypox virus infection diagnosis or differential diagnosis.
-
Product Information
Package Size: 24 tests/kit, 100 tests/kit
-
Sample Requirements
Sample Type: Serum samples or skin lesion samples: including lesion rash, pustule surface and/or exudate swabs, pustule fluid, pustule epidermis or scab.
-
Storage Conditions and Validity Period
Storage Conditions: -20±5℃
Validity Period: 12 months